Src Kinases
The discovery in 1976 that the v-src oncogene of the Rous Sarcoma Virus has a normal cellular homolog (c-src) defined the existence of proto-oncogenes in the normal cellular genome, and laid the foundation for the hypothesis that human cancers may arise due to the activation of cellular proto-oncogenes. Over the subsequent decades many more retroviral oncogenes were discovered and their cellular proto-oncogene counterparts were discovered. In the era of tumor genomic sequencing many of these proto-oncogenes were found to be genetically altered and functionally activated, validating the original oncogene hypothesis of human cancer pathogenesis. Many of these cancers are now effectively treated by inhibitors of their driving oncogenes, closing the loop on the oncogene hypothesis and demonstrating the power of scientific research to impact human health and disease mortality.
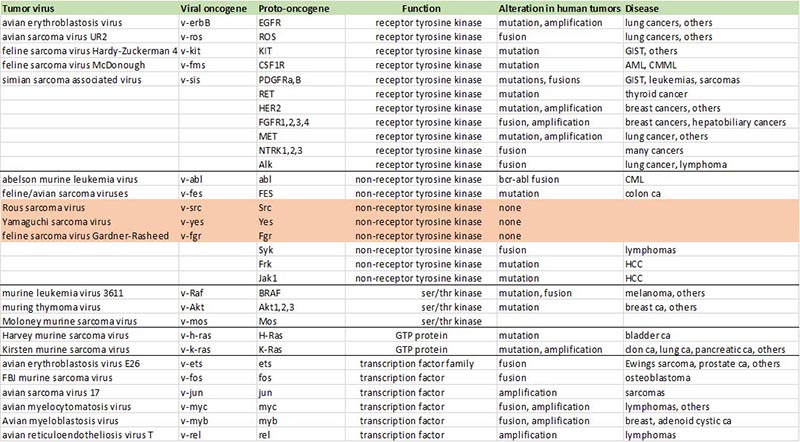
But the hypothesis remains unproven for c-src itself, or any of the Src family members, and despite decades of studies, there is little evidence that human cancers arise due to the activation of the c-src proto-oncogene. The mutational activation of Src kinases is not seen in human tumors, and although an increase in expression and associated activities of Src and Yes are seen in many epithelial cancers, Src kinases are not transforming through overexpression alone, and the attribution of a tumor-driving function to these kinases remains a weakly supported notion at this time. Several classes of Src-selective tyrosine kinase inhibitors have now been tested in clinical studies showing little evidence of clinical efficacy. Although the tumorigenic role of the retroviral v-src in generating chicken sarcomas is clear, the role of cellular Src kinases in human cancers remains largely unknown. As such, the 1970s idea that the proto-oncogene c-src is activated to an oncogene in human cancers is now conceptually deadlocked.
We are interested in understanding how, if at all, the functions of Src kinases are perturbed in human cancers. We believe that the mode of function of Src kinases is not well understood, and this has limited our ability to understand its mode of dysfunction in human cancers. We are studying how Src kinases select substrates, the temporal regulation of substrate selection, and the structural basis for substrate selection. In particular, since much of our understanding of the mode of regulation of Src comes from crystallographic studies, the role of the unstructured N-terminal unique domain of Src has been poorly understood. Recent evidence suggests that unstructured regions of proteins are critically involved in their regulation, and are particularly targeted by post-translational modifications. We have shown that although the Src unique domain mediates membrane binding, it also mediates Src dimerization, potentially creating a conformational landscape that can be regulated by post-translational modifications.